Names of aldehydes and ketones. Carbonyl group. Aldehydes and ketones. Preparation of aldehydes and ketones
Aldehydes and ketones are carbonyl organic compounds. Carbonyl compounds are organic substances whose molecules contain a >C=O group (carbonyl or oxo group).
General formula of carbonyl compounds:
The functional group –CH=O is called aldehyde. Ketones- organic substances whose molecules contain a carbonyl group connected to two hydrocarbon radicals. General formulas: R 2 C=O, R–CO–R" or
|
|
|
Models of the simplest carbonyl compounds |
||
|
Name | ||
|
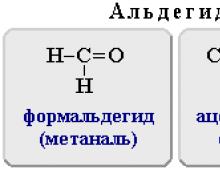
Formaldehyde (methanal) |
H 2 C=O | |
|
Acetaldehyde (ethanal) |
CH 3 -CH=O | |
|
Acetone (propanone) |
(CH 3 ) 2 C=O |
|
Nomenclature of aldehydes and ketones.
Systematic names aldehydes built by the name of the corresponding hydrocarbon and adding a suffix -al. Chain numbering begins with the carbonyl carbon atom. Trivial names are derived from the trivial names of those acids into which aldehydes are converted during oxidation.
|
Formula |
Name |
|
|
systematic |
trivial |
|
|
H 2 C=O |
methane al |
formic aldehyde (formaldehyde) |
|
CH 3 CH=O |
ethane al |
acetaldehyde (acetaldehyde) |
|
(CH 3 ) 2 CHCH=O |
2-methylpropane al |
isobutyraldehyde |
|
CH 3 CH=CHCH=O |
butene-2- al |
crotonaldehyde |
Systematic names ketones simple structure is derived from the names of radicals (in increasing order) with the addition of the word ketone. For example: CH 3 –CO–CH 3 - dimethyl ketone(acetone); CH 3 CH 2 CH 2 –CO–CH 3 - methylpropyl ketone. More generally, the name of a ketone is based on the name of the corresponding hydrocarbon and the suffix -He; Chain numbering starts from the end of the chain closest to the carbonyl group (IUPAC substitutive nomenclature). Examples: CH 3 –CO–CH 3 - propane He(acetone); CH 3 CH 2 CH 2 –CO–CH 3 - pentane He- 2; CH 2 =CH–CH 2 –CO–CH 3 - pentene-4 -He- 2.
Isomerism of aldehydes and ketones.
Aldehydes and ketones are characterized by structural isomerism.
Isomerism aldehydes:
|
isomerism of the carbon skeleton, starting with C 4 |
|
|
interclass isomerism with ketones, starting with C 3 |
|
|
cyclic oxides (with C 2) |
|
|
unsaturated alcohols and ethers (with C 3) |
|
|
Isomerism ketones: carbon skeleton (c C 5) |
|
|
position of the carbonyl group (c C 5) |
|
interclass isomerism (similar to aldehydes).
Structure of the carbonyl group C=O.
The properties of aldehydes and ketones are determined by the structure of the carbonyl group >C=O.

The C=O bond is highly polar. Its dipole moment (2.6-2.8D) is significantly higher than that of the C–O bond in alcohols (0.70D). The electrons of the C=O multiple bond, especially the more mobile -electrons, are shifted towards the electronegative oxygen atom, which leads to the appearance of a partial negative charge on it. The carbonyl carbon acquires a partial positive charge.

Therefore, carbon is attacked by nucleophilic reagents, and oxygen is attacked by electrophilic reagents, including H +.
The molecules of aldehydes and ketones lack hydrogen atoms capable of forming hydrogen bonds. Therefore, their boiling points are lower than those of the corresponding alcohols. Methanal (formaldehyde) is a gas, aldehydes C 2 -C 5 and ketones C 3 -C 4 are liquids, higher substances are solids. Lower homologues are soluble in water due to the formation of hydrogen bonds between the hydrogen atoms of water molecules and the carbonyl oxygen atoms. As the hydrocarbon radical increases, solubility in water decreases.
Reaction centers of aldehydes and ketones
sp 2 -The hybridized carbon atom of the carbonyl group forms three σ bonds lying in the same plane and a π bond with the oxygen atom due to the unhybridized p orbital. Due to the difference in electronegativity of carbon and oxygen atoms, the π bond between them is highly polarized (Fig. 5.1). As a result, a partial positive charge δ+ appears on the carbon atom of the carbonyl group, and a partial negative charge δ- appears on the oxygen atom. Since the carbon atom is electron deficient, it provides a site for nucleophilic attack.
Distribution of electron density in molecules of aldehydes and ketones, taking into account the transfer of electronic influence by electron-

Rice. 5.1. Electronic structure of the carbonyl group
the deficient carbon atom of the carbonyl group along σ-bonds is presented in Scheme 5.1.
Scheme 5.1. Reaction centers in the molecule of aldehydes and ketones

There are several reaction centers in the molecules of aldehydes and ketones:
The electrophilic center - the carbon atom of the carbonyl group - determines the possibility of nucleophilic attack;
The main center - the oxygen atom - makes it possible to attack with a proton;
A CH acid center whose hydrogen atom has weak proton mobility and can, in particular, be attacked by a strong base.
In general, aldehydes and ketones are highly reactive.
Among oxygen-containing organic compounds, two classes of substances are of great importance, which are always studied together for their similarity in structure and properties. These are aldehydes and ketones. It is these molecules that underlie many chemical syntheses, and their structure is interesting enough to become the subject of study. Let's take a closer look at what these classes of compounds are.
Aldehydes and ketones: general characteristics
From a chemical point of view, the class of aldehydes should include organic molecules containing oxygen as part of the functional group -SON, called carbonyl. The general formula in this case will look like this: R-COH. By their nature, these can be both limiting and non-limiting compounds. Also among them there are aromatic representatives, along with aliphatic ones. The number of carbon atoms in the radical chain varies quite widely, from one (formaldehyde or methanal) to several dozen.
Ketones also contain a carbonyl group -CO, but it is not connected to a hydrogen cation, but to another radical, different or identical to the one in the chain. The general formula looks like this: R-CO-R, . It is obvious that aldehydes and ketones are similar in the presence of a functional group of this composition.
Ketones can also be saturated and unsaturated, and the properties exhibited are similar to those of a closely related class. Several examples can be given to illustrate the composition of molecules and reflect the accepted designations for the formulas of the substances in question.
- Aldehydes: methanal - HCOH, butanal - CH 3 -CH 2 -CH 2 -CH, phenylacetic - C 6 H 5 -CH 2 -CH.
- Ketones: acetone or dimethyl ketone - CH 3 -CO-CH 3, methyl ethyl ketone - CH 3 -CO-C 2 H 5 and others.
Obviously, the name of these compounds is formed in two ways:
- according to rational nomenclature according to the radicals included in the composition and the class suffix -al (for aldehydes) and -on (for ketones);
- trivial, historically established.
If we give the general formula for both classes of substances, it will become clear that they are isomers of each other: C n H 2n O. They themselves are characterized by the following types of isomerism:

To distinguish between representatives of both classes, qualitative reactions are used, most of which allow the identification of the aldehyde. Since the chemical activity of these substances is slightly higher, due to the presence of a hydrogen cation.
Molecule structure
Let's look at what aldehydes and ketones look like in space. The structure of their molecules can be reflected in several points.
- The carbon atom directly included in the functional group has sp 2 hybridization, which allows part of the molecule to have a flat spatial shape.
- In this case, the polarity of the C=O bond is strong. Being more electronegative, oxygen takes the bulk of the density, concentrating a partially negative charge on itself.
- In aldehydes, the O-H bond is also highly polarized, which makes the hydrogen atom mobile.
As a result, it turns out that such a structure of molecules allows the compounds in question to be both oxidized and reduced. The formula of an aldehyde and a ketone with a redistributed electron density makes it possible to predict the products of reactions in which these substances participate.
History of discovery and study
Like many organic compounds, people succeeded in isolating and studying aldehydes and ketones only in the 19th century, when vitalistic views completely collapsed and it became clear that these compounds could be formed synthetically, artificially, without the participation of living beings.
However, back in 1661, R. Boyle managed to obtain acetone (dimethyl ketone) when he exposed calcium acetate to heat. But he could not study this substance in detail and name it, determine its systematic position among others. It was only in 1852 that Williamson was able to bring this matter to completion, and then the history of the detailed development and accumulation of knowledge about carbonyl compounds began.

Physical properties
Let's look at the physical properties of aldehydes and ketones. Let's start with the first ones.
- The first representative of methanal in its state of aggregation is a gas, the next eleven are liquids, over 12 carbon atoms are part of solid aldehydes of normal structure.
- Boiling point: depends on the number of C atoms; the more there are, the higher it is. In this case, the more branched the chain, the lower the temperature drops.
- For liquid aldehydes, the viscosity, density, and refractive indexes also depend on the number of atoms. The more there are, the higher they are.
- Gaseous and liquid aldehydes dissolve in water very well, but solid ones practically cannot do this.
- The smell of representatives is very pleasant, often the aromas of flowers, perfumes, and fruits. Only those aldehydes in which the number of carbon atoms is 1-5 are strong and unpleasant-smelling liquids.
If we denote the properties of ketones, we can also highlight the main ones.
- Aggregate states: lower representatives are liquids, more massive ones are solid compounds.
- The smell is pungent and unpleasant in all representatives.
- Solubility in water is good for the lower ones, and excellent in organic solvents for all.
- Volatile substances, this indicator exceeds that of acids and alcohols.
- The boiling and melting points depend on the structure of the molecule and vary greatly depending on the number of carbon atoms in the chain.
These are the main properties of the compounds under consideration, which belong to the group of physical ones.

Chemical properties
The most important thing is what aldehydes and ketones react with and the chemical properties of these compounds. Therefore, we will definitely consider them. First, let's deal with aldehydes.
- Oxidation to the corresponding carboxylic acids. The general form of the reaction equation is: R-COH + [O] = R-COOH. Aromatic representatives enter into such interactions even more easily, and they are also capable of forming esters, which are of great industrial importance. The following oxidizing agents are used: oxygen, Tollens' reagent, copper (II) hydroxide and others.
- Aldehydes manifest themselves as strong reducing agents, while turning into saturated monohydric alcohols.
- Interaction with alcohols to form acetals and hemiacetals.
- Special reactions are polycondensation. As a result, phenol-formaldehyde resins are formed, which are important for the chemical industry.
- Several specific reactions with the following reagents:
- hydroalcoholic alkali;
- Grignard reagent;
- hydrosulfites and others.
A qualitative reaction to this class of substances is the “silver mirror” reaction. As a result, metallic reduced silver and the corresponding carboxylic acid are formed. It requires an ammonia solution of silver oxide or Tollins reagent.

Chemical properties of ketones
Alcohols, aldehydes, and ketones are compounds with similar properties, since they are all oxygen-containing. However, already at the oxidation stage it becomes clear that alcohols are the most active and easily affected compounds. Ketones are the most difficult to oxidize.
- Oxidative properties. As a result, secondary alcohols are formed.
- Hydrogenation also leads to the products mentioned above.
- Keto-enol tautomerism is a special specific property of ketones to take the beta form.
- Aldol condensation reactions with the formation of beta-keto alcohols.
- Ketones can also interact with:
- ammonia;
- hydrocyanic acid;
- hydrosulfites;
- hydrazine;
- orthosilicic acid.
Obviously, the reactions of such interactions are very complex, especially those that are specific. These are all the main features that aldehydes and ketones exhibit. Chemical properties underlie many syntheses of important compounds. Therefore, knowing the nature of molecules and their character during interactions is extremely necessary in industrial processes.
Addition reactions of aldehydes and ketones
We have already examined these reactions, but did not give them such a name. All interactions as a result of which the carbonyl group exhibited activity can be classified as addition. Or rather, a mobile hydrogen atom. That is why in this matter preference is given to aldehydes, due to their better reactivity.
With what substances are reactions of aldehydes and ketones possible by nucleophilic substitution? This:
- Hydrocyanic acid produces cyanohydrins - the starting material for the synthesis of amino acids.
- Ammonia, amines.
- Alcohols.
- Water.
- Sodium hydrogen sulfate.
- Grignard reagent.
- Thiols and others.
These reactions are of great industrial importance, since the products are used in various areas of human activity.

Methods of obtaining
There are several main methods by which aldehydes and ketones are synthesized. Production in laboratory and industry can be expressed in the following ways.
- The most common method, including in laboratories, is the oxidation of the corresponding alcohols: primary to aldehydes, secondary to ketones. The following can act as an oxidizing agent: chromates, copper ions, potassium permanganate. General form of the reaction: R-OH + Cu (KMnO 4) = R-COH.
- In industry, a method based on the oxidation of alkenes - oxosynthesis - is often used. The main agent is synthesis gas, a mixture of CO 2 + H 2. The result is an aldehyde with one more carbon in the chain. R=R-R + CO 2 + H 2 = R-R-R-COH.
- Oxidation of alkenes with ozone - ozonolysis. The result also suggests an aldehyde, but also a ketone in the mixture. If the products are mentally combined by removing the oxygen, it will become clear which original alkene was taken.
- Kucherov reaction - hydration of alkynes. An obligatory agent is mercury salts. One of the industrial methods for the synthesis of aldehydes and ketones. R≡R-R + Hg 2+ + H 2 O = R-R-COH.
- Hydrolysis of dihalogenated hydrocarbons.
- Reduction of: carboxylic acids, amides, nitriles, acid chlorides, esters. As a result, both an aldehyde and a ketone are formed.
- Pyrolysis of mixtures of carboxylic acids over catalysts in the form of metal oxides. The mixture should be steamy. The essence is the splitting between carbon dioxide and water molecules. As a result, an aldehyde or ketone is formed.
Aromatic aldehydes and ketones are prepared by other methods, since these compounds have an aromatic radical (phenyl, for example).
- According to Friedel-Crafts: the starting reagents contain an aromatic hydrocarbon and a dihalogenated ketone. Catalyst - ALCL 3. As a result, an aromatic aldehyde or ketone is formed. Another name for the process is acylation.
- Oxidation of toluene by the action of various agents.
- Reduction of aromatic carboxylic acids.
Naturally, industry tries to use those methods in which the feedstock is as cheap as possible and the catalysts are less toxic. For the synthesis of aldehydes, this is the oxidation of alkenes with oxygen.

Industrial Applications and Significance
The use of aldehydes and ketones is carried out in such industries as:
- pharmaceuticals;
- chemical synthesis;
- medicine;
- perfume area;
- food industry;
- paint and varnish production;
- synthesis of plastics, fabrics, etc.
It is possible to identify more than one area, because approximately 6 million tons of formaldehyde alone are synthesized annually! Its 40% solution is called formalin and is used for storing anatomical objects. It is also used for the production of medicines, antiseptics and polymers.
Acetaldehyde, or ethanal, is also a mass-produced product. The amount of annual consumption in the world is about 4 million tons. It is the basis of many chemical syntheses in which important products are formed. For example:
- acetic acid and its anhydride;
- cellulose acetate;
- medicines;
- butadiene - the basis of rubber;
- acetate fiber.
Aromatic aldehydes and ketones are components of many flavorings, both food and perfume. Most of them have very pleasant floral, citrus, herbal aromas. This makes it possible to produce on their basis:
- air fresheners of various kinds;
- toilet and perfume waters;
- various cleaning products and detergents.
Some of them are aromatic food additives approved for consumption. Their natural content in essential oils, fruits and resins proves the possibility of such use.
Individual representatives
An aldehyde such as citral is a liquid with high viscosity and a strong lemon aroma. It is found in nature in essential oils of the latter. Also contains eucalyptus, sorghum, kebab.
Its areas of application are well known:
- pediatrics - decreased intracranial pressure;
- normalization of blood pressure in adults;
- component of a medicine for the organs of vision;
- an integral part of many aromatic substances;
- anti-inflammatory and antiseptic;
- raw materials for the synthesis of retinol;
- flavoring for food purposes.
|
T Omsk State University |
||
|
Department of Organic Chemistry |
||
|
Aldehydes and ketones |
||
Aldehydes and ketones are distinguished by the presence of a carbonyl group >C=Oh.
The carbonyl group is bond polarized S-O:
Aldehydes and ketones can be considered as derivatives alkanes, who have one of methyl (-CH 3) or methylene groups ( -CH 2 - ) is replaced by a carbonyl group:

Ketones have two alkyl radicals as substituents on the carbonyl group, while aldehydes have one substituent b- alkyl group, the other is hydrogen. This difference leads to significant differences in chemical properties ( cm. below).
Nomenclature
NomenclatureIUPAC
When naming aldehydes and ketones according to the IUPAC nomenclature rules, the longest carbon chain containing the carbonyl group is selected. The numbering of carbon atoms in this chain is carried out from the end where the carbonyl group is closest, and when forming the name to the name of the hydrocarbon corresponding to the number of carbon atoms in the main chain (1-methane, 2-ethane, 3-propane, 4-butane, 5 - pentane, etc.) the ending is added -A l (for aldehydes) or -He for ketones.
The position of the carbonyl group in ketones is indicated by a dash if multiple isomers are possible. The position of the carbonyl group of aldehydes is not indicated by a number, since in all cases it appears under the first number:

Rational nomenclature
Ketones are often named after the radicals connected through the carbonyl group, with the addition of the word ketone. For example, hexanone-3 or methylethyl ketone , acetone or dimethyl keto n.
Aldehydes can be named as derivatives ethanal or acetaldehyde:
Other name e - trimethylethanal.
Chemical properties of carbonyl compounds
All reactions of carbonyl compounds can be divided into groups:
Reactions on the carbonyl group (addition)
Reactions by carbon skeleton
Oxidation reactions
Recovery reactions
Addition reactions at the carbonyl group (addition of nucleophilic reagents)
1. water connection

Emerging heme diols are unstable and the equilibrium in this reaction is strongly shifted to the left. The exception is aldehydes and ketones with electron-withdrawing groups, for example, chloral or hexafluoroacetone, which in the aquatic environment exist in the form heme diols:

2. addition of bisulfite

The addition occurs through the more nucleophilic sulfur atom rather than the oxygen atom, although it has a negative charge. Derivatives are formed alkanesulfonic acids(salts alkaneoxysulfonic acids).
Emerging adducts insoluble in saturated sodium bisulfite solution or alcohols and precipitates in the form of crystals. This way you can separate carbonyl compounds from a mixture with alcohols. The carbonyl compound is released in free form from adduct when treated with acid.
When reacting with ketones, bisulfites add only to methyl ketones CH 3 -CO-R.
3. addition of cyanide

The reaction is catalyzed by potassium cyanide or sodium cyanide. Emerging oxynitriles(or cyanohydrins) can be hydrolyzed before oxycarbonic acids:
4. addition of alcohols
Upon addition of the first alcohol molecule, hemiacetals. The reaction is catalyzed by acids or bases:

The addition of a second alcohol molecule results in the formation acetals. Education acetals catalyzed only in acidic medium:

Acetalsstable in neutral and alkaline environments, therefore they can be used for temporary protection of aldehyde groups. Acetals wide common in nature.
5. connection of reagents Grignard
Interaction of organometallic compounds type R-Mg-X(reagents Grignard), where X = halogen, with carbonyl groups (nucleophilic addition at a multiple bond WITH=O):

Interaction formaldehyde, aldehydes, ketones And - leads to primary, secondary and tertiary alcohols, respectively.
Tertiary alcohols are obtained from ketones. Yes, from methyl ethyl ketone(butanone-2) produces 2-methylbutanol-2. Aldehydes in a similar reaction give secondary alcohols. From propionic aldehyde ( propanal) butanol-2 is obtained:
Primary alcohols are formed from formaldehyde. When reactants interact Grignard With acid halides carboxylic acids and esters form tertiary alcohols, which have two identical alkyl substituents. This consumes two moles of reagent Grignard:

6. Addition of ammonia and amines
Primary amines combine with aldehydes and ketones to formimins (reasons Schiffa :

A similar reaction of secondary amines with carbonyl compounds gives enamines :

Hydrazine and its derivatives can also react with carbonyl compounds to form hydrazones:

Hydroxylamines combine with aldehydes and ketones to form aldoximes And ketoximes:

7. Aldol-crotonic condensation
Condensation can occur in both acidic and alkaline environments.
Acid catalyzed condensation
They enter into condensation enol And protonated carbonyl group of the second molecule of the compound:
Base catalyzed condensation
Education enolate ion, generating
carbanion, proceeds according to the scheme:
Further carbanion attaches to the carbonyl group of the second molecule, and proceeds C-alkylation, Unlike thermodynamically disadvantageous ABOUT- alkylation:
Emerging aldehyde alcohol (aldol) easily loses water in the presence of catalytic amounts of bases or acids, as well as with slight heating, with the formation of a,b - unsaturated carbonyl compound, this completes the condensation reaction (R,X = alkyl or H):

Thus, in the reaction aldoln O- croton condensation (including self-condensation) both aldehydes and ketones can enter, having alpha carbons hydrogen atoms. In the case of ketones, the equilibrium position is unfavorable for the formation of products; however, by carrying out the reaction under special conditions (for example, excluding contact of the product with the main catalyst), significant yields can be achieved. Cross-reactions between aldehydes and ketones have no laboratory application because they form difficult to separate a mixture of four products and unreacted starting compounds. More often, for synthetic purposes, a reaction is carried out between two carbonyl compounds, one of which is a source of carbanions ( methylene component ), and the other serves carbonyl component (without alpha carbon hydrogen atoms). Typically, formaldehyde, aromatic aldehydes, esters of carbonic, oxalic and formic acids are used as the carbonyl component. C-H acids and even derivatives of acetylene hydrocarbons with a terminal triple bond are used as the methylene component.
8. Cannizzaro's reaction
Aldehydes that do not have alpha carbon Hydrogen atoms, when heated, with strong bases enter into an oxidation-reduction reaction, when one of the molecules is reduced to alcohol due to the oxidation of the second molecule to a carboxylic acid. Such reactions are called Cannizzaro's reactions, and proceed according to the scheme:

Intramolecular oxidation-reduction reactions are also known:

With a peculiar type of intramolecular oxidation-reduction is Benzyl regrouping :
Reactions on the carbon skeleton of aldehydes and ketones
Reactions affecting the carbon skeleton include:
Keto-enol tautomerism of aldehydes and ketones;
Halogenation (haloform reaction and replacement of a-carbon hydrogen atoms)
1. Keto-enol tautomerism
Carbonyl compounds can coexist in two forms - ketone and enol:

The transformation of aldehydes and ketones into enols (unsaturated alcohols) occurs both spontaneously and with catalysis by acids and bases. Enol forms, although present in aldehydes and ketones in insignificant concentrations, play a significant role in their reactivity. A number of important reactions of aldehydes and ketones occur through the formation of enols. Let us consider the mechanisms of the transition of ketone forms to enol forms, which occur under the catalytic action of acids and bases.
Enolization acid catalyzed
The formation of enol can be catalyzed by an acid according to the scheme below (R" = alkyl or H):
The reaction begins with the protonation of the oxygen atom of the carbonyl group and ends with the removal of a proton from alpha carbon atom. Thus, formally the proton plays the role of a catalyst.
Enolization , catalyzed basis
The formation of enolate ion proceeds according to the following scheme:
The acidity of the alpha carbon hydrogen atoms plays an important role in the formation of enols catalyzed by bases. Their increased acidity is associated with the close proximity to the carbonyl group and its negative inductive effect, which withdraws electrons from the C-H bond and thus facilitates proton abstraction. In other words, proton abstraction is facilitated because the resulting carbanion is stabilized by delocalization of the negative charge onto the carbonyl group.
Halogens are added to the resulting enols via a multiple C = C bond. Only unlike alkenes, where such addition is completed by complete binding of the halogen, in aldehydes and ketones only one halogen atom is added (on the carbon adjacent to the carbonyl group). The second halogen atom (on the carbonyl group) is not added, and the reaction ends with the removal of a proton and regeneration of the carbonyl group:
In an acidic environment the reaction stops there. The second hydrogen atom is not replaced by a halogen. But in an alkaline environment, a rapid reaction of substitution of the second, and an even faster reaction of substitution of the third carbon atom with a halogen occurs (an increase in the number of halogen atoms at carbon sharply increases the acidity of its hydrogens):
Ultimately, all three hydrogen atoms are replaced by halogens, followed by elimination of the group CX 3 as an anion, followed by immediate proton exchange:
As a result, trihalomethane, called haloform (iodoform CHJ 3, bromoform CHBr 3, chloroform CHCl 3) and carboxylic acid anion. And the process itself is called a haloform reaction. Any methyl ketones are susceptible to the haloform reaction. Haloforms precipitate as a colored precipitate (yellow iodoform), have a specific odor and can serve as a qualitative reaction to the presence of methyl ketones. Alcohols also give a haloform reaction, the oxidation of which can form methyl ketones (for example, isopropanol). Oxidation is carried out by an excess amount of halogen.
Oxidation of aldehydes and ketones
Aldehydes are easily oxidized to the corresponding acids:
Ketones are difficult to oxidize under harsh conditions. Oxidation is accompanied by the cleavage of the C-C bond adjacent to the carbonyl group. The result is a set of oxidation products - carboxylic acids with different carbon chain lengths:

Methods receiving
1. Oxidation primary alcohols produce aldehydes, and secondary alcohols produce ketones:

Oxidation can be carried out using “dry” and “wet” methods. The first is to pass alcohol vapor through a heated to 300-350 WITH copper oxide CuO. The “wet” method is the oxidation of alcohols with an acidified solution of potassium or sodium bichromate:
When oxidizing by the “wet” method, the resulting aldehyde should be distilled off from the reaction sphere, otherwise it is easily oxidized further to carboxylic acid:
2. Aldehydes and ketones are obtained with hydrolysis heme-dihaloalkanes

First, two halogen atoms are replaced by hydroxyl groups. But unstable heme diols quickly rearrange into carbonyl compounds with the elimination of a water molecule:
3. Ozonolysis
alkenes
leads to the formation of mixtures of aldehydes and ketones, depending on the structure of the starting material alkene:
At the first stage of ozonation, ozonide is obtained, the decomposition of which with water produces carbonyl compounds and hydrogen peroxide. To prevent peroxide from provoking further oxidation of aldehydes, zinc dust is added to the water during the decomposition of ozonides. Ozonation of alkenes is aimed not so much at the synthesis of aldehydes and ketones, but at determining the location of the multiple bond:

4. Addition of water to alkynes
The addition of water to a triple bond in the presence of mercury salts leads to acetaldehyde in the case of acetylene, and to ketones in the case of substituted acetylenes. Waterjoins according to Markovnikov's rule:
It's time to get to know this class of organic compounds in more detail.
\
Aldehydes
- organic substances whose molecules contain a carbonyl group C=0 connected to a hydrogen atom and a hydrocarbon radical. /
The general formula of aldehydes is
Organic substances in whose molecules a carbonyl group is linked to two hydrocarbon radicals are called ketones.
Obviously, the general formula of ketones is
O
II
R1-C-R2
The carbonyl group of ketones is called the keto group.
In the simplest ketone, acetone, the carbonyl group is linked to two methyl radicals:
O
II
CH3-C-CH3
Nomenclature and isomerism
Depending on the structure of the hydrocarbon radical associated with the aldehyde group, saturated, unsaturated, aromatic, heterocyclic and other aldehydes are distinguished. According to the IUPAC nomenclature, the names of saturated aldehydes are formed from the name of an alkane with the same number of carbon atoms in the molecule using the suffix -al.
The numbering of the carbon atoms of the main chain begins with the carbon atom of the aldehyde group. Therefore, the aldehyde group is always located at the first carbon atom, and there is no need to indicate its position with a number.
Along with systematic nomenclature, trivial names of widely used aldehydes are also used. These names are usually derived from the names of carboxylic acids corresponding to aldehydes.
To name ketones according to systematic nomenclature, the keto group is designated by the suffix -one and a number that indicates the number of the carbon atom of the carbonyl group (numbering should begin from the end of the chain closest to the keto group).
Aldehydes are characterized by only one type of structural isomerism - isomerism of the carbon skeleton, which is possible with butanal, and for ketones also isomerism of the position of the carbonyl group (write down the structural formulas of butanone isomers and name them). In addition, they are characterized by interclass isomerism (propanal and propanone). 
Physical properties
In an aldehyde or ketone molecule, due to the greater electronegativity of the oxygen atom compared to the carbon atom, the C=0 bond is highly polarized due to a shift in the electron density P-bonds to oxygen.
Aldehydes and ketones are polar substances with excess electron density on the oxygen atom. The lower members of the series of aldehydes and ketones (formaldehyde, acetaldehyde, acetone) are unlimitedly soluble in water. Their boiling points are lower than those of the corresponding alcohols (see Table 5). This is due to the fact that in the molecules of aldehydes and ketones, unlike alcohols, there are no mobile hydrogen atoms and they do not form associates due to hydrogen bonds. Lower aldehydes have a pungent odor; aldehydes containing four to six carbon atoms in the chain have an unpleasant odor; higher aldehydes and ketones have floral odors and are used in perfumery.
Chemical properties of saturated aldehydes and ketones
The presence of an aldehyde group in a molecule determines the characteristic properties of aldehydes.
Recovery reactions
The addition of hydrogen to aldehyde molecules occurs through the double bond in the carbonyl group. The product of hydrogenation of aldehydes is primary alcohols, and ketones are secondary alcohols. Thus, when hydrogenating acetaldehyde on a nickel catalyst, ethyl alcohol is formed, and when hydrogenating acetone, 2-propanol is formed.
Hydrogenation of aldehydes is a reduction reaction in which the oxidation state of the carbon atom included in the carbonyl group decreases.
Oxidation reactions
Aldehydes can not only be reduced, but also oxidized. When oxidized, aldehydes form carboxylic acids. This process can be schematically represented as follows:
![]()
From propionic aldehyde (propanal), for example, propionic acid is formed:

If the surface of the vessel in which the reaction is carried out has been previously degreased, then the silver formed during the reaction covers it with a thin, even film. This makes a wonderful silver mirror. Therefore, this reaction is called the “silver mirror” reaction. It is widely used for making mirrors, silvering decorations and Christmas tree decorations.
Freshly precipitated copper(II) hydroxide can also act as an oxidizing agent for aldehydes. Oxidizing the aldehyde, Cu2+ is reduced to Cu4. The copper(I) hydroxide CuOH formed during the reaction immediately decomposes into red copper(I) oxide and water.
This reaction, as well as the "silver mirror" reaction, is used to detect aldehydes.
Ketones are not oxidized either by atmospheric oxygen or by such a weak oxidizing agent as an ammonia solution of silver oxide.
Addition reactions
Since the carbonyl group contains a double bond, aldehydes and ketones are able to undergo addition reactions. The C=0 bond is polar; a partial positive charge is concentrated on the carbon atom. Aldehydes and ketones undergo nucleophilic addition reactions. Such reactions begin with the interaction of a carbon atom of a carbonyl group with a free electron pair of a nucleophilic reagent (Nu). The resulting anion then adds a proton or another cation.
The nucleophilic addition of hydrocyanic acid in the presence of traces of alkalis to aldehydes and ketones produces oxynitriles (cyanohydrins). Aldehydes and methyl ketones react nucleophilically with sodium hydrosulfite.
The resulting hydrosulfite derivatives of aldehydes and ketones decompose when heated with mineral acids or soda to form the original carbonyl compounds.
Aldehydes and ketones are capable of adding organomagnesium compounds (Grignard reagents). These compounds are prepared by reacting magnesium metal with a haloalkane in absolute (anhydrous) diethyl ether.
The hydrocarbon radical R of an organomagnesium compound, on which a partial negative charge is concentrated, nucleophilically attaches to the carbon atom of the carbonyl group, and the MgX residue attaches to the oxygen atom:
After decomposition of the resulting product with an aqueous acid solution, alcohol is formed.
Using this reaction, a primary alcohol can be obtained from formaldehyde, a secondary alcohol can be obtained from any other aldehyde, and a tertiary alcohol can be obtained from a ketone. For example, 2-butanol can be obtained from acetaldehyde and ethylmagnesium bromide.
Aldehydes and ketones react with halogens in a substitution reaction, even in the absence of light. In this case, only the hydrogen atoms at the carbon atom adjacent to the carbonyl group are replaced by halogen.
What causes the selectivity of the halogenation of carbonyl compounds? It can be assumed that the reason for such selectivity of substitution is the mutual influence of groups of atoms on each other. Indeed, aldehydes and ketones containing hydrogen atoms at the carbon atom adjacent to the carbonyl group are capable of isomerizing into unsaturated alcohols - enols. The substitution reaction by the ionic mechanism includes an intermediate stage - the formation of the enol form of an aldehyde or ketone.
Aldehydes undergo a polycondensation reaction. Studying phenols, we examined in detail the interaction of methanal (formaldehyde) with phenol (§ 18), leading to the formation of phenol-formaldehyde resins.
Methods of obtaining
Aldehydes and ketones can be prepared by oxidation or dehydrogenation of alcohols. Let us note once again that the oxidation or dehydrogenation of primary alcohols can produce aldehydes, and that of secondary alcohols - ketones.
The Kucherov reaction (hydration of alkynes) is discussed in § 13. Let us recall that the reaction produces acetaldehyde from acetylene, and ketones from acetylene homologues:

Individual representatives of aldehydes and their significance
Formaldehyde (methanal, formic aldehyde) HCHO is a colorless gas with a pungent odor and a boiling point of -21 ° C, highly soluble in water. Formaldehyde is poisonous! A solution of formaldehyde in water (40%) is called formalin and is used for disinfection. In agriculture, formaldehyde is used to treat seeds, and in the leather industry - for treating leather. Formaldehyde is used to produce methenamine, a medicinal substance. Sometimes methenamine compressed in the form of briquettes is used as fuel (dry alcohol). A large amount of formaldehyde is consumed in the production of phenol-formaldehyde resins and some other substances.
Acetaldehyde (ethanal, acetaldehyde) CH 3 CHO is a liquid with a pungent, unpleasant odor and a boiling point of 21 ° C, highly soluble in water. Acetic acid and a number of other substances are produced from acetaldehyde on an industrial scale; it is used for the production of various plastics and acetate fiber. Acetaldehyde is poisonous!
1. How many carbon atoms are there in a molecule of the simplest aldehyde? in the simplest ketone molecule? Name these substances. Give synonyms for their names.
2. Name the substances whose structural formulas are as follows: 
3. Write down the structural formulas of butanal isomers. What classes do these substances belong to? Name them. Write down equations for the hydrogenation reactions of these compounds and indicate the names of the reaction products.
4. What volume of formaldehyde (n.o.) must be hydrogenated to obtain 16 g of methyl alcohol?
5. Write an equation for the hydrogenation reaction of dimethyl ketone (acetone). What is the molar mass of the reaction product?
6. Write down the equation for the “silver mirror” reaction involving methanal. What functional groups does the carboxylic acid molecule, the product of this reaction, contain? Can it be oxidized with an ammonia solution of silver oxide? What can be formed in this case? Illustrate your answer with reaction equations.
7. During the “silver mirror” reaction, a carboxylic acid was formed with a relative molecular weight of 88. What organic substances could be reagents in this reaction? Using structural formulas, create possible equations for this reaction.
8. What mass of acetaldehyde is needed to reduce 0.54 g of silver from its oxide? What amount of potassium hydroxide is needed to neutralize the acetic acid formed?
9. In one of the vessels there is a solution of acetone, in the other - acetaldehyde. Suggest ways to determine the contents of each container.
10. What substances are formed when copper(II) hydroxide is heated with propanal? Support your answer with the reaction equation. What are the signs of this reaction?
11. The combustion of 4.5 g of organic matter produced 3.36 liters (n.s.) of carbon dioxide and 2.7 ml of water. Determine the simplest and true formula of a substance if its density in air is 1.035. Explain the etymology of the names of this substance. What are its areas of application?
12*. Write down equations for the reactions that can occur during the bromination of propanal in the light. What products can be formed in this case? Name them. What products are formed when propanal reacts with acidified bromine water? Name them.
13*. The oxidation of 11.6 g of an oxygen-containing organic compound resulted in the formation of 14.8 g of monobasic carboxylic acid, which reacted with excess sodium bicarbonate to release 4.48 liters of gas. Determine the structure of the original compound.
14*. When 1.18 g of a mixture of formic and acetaldehydes was oxidized with an excess of ammonia solution of silver oxide, 8.64 g of precipitate was formed. Determine the mass fractions of aldehydes in the mixture.
Lesson content lesson notes supporting frame lesson presentation acceleration methods interactive technologies Practice tasks and exercises self-test workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures, graphics, tables, diagrams, humor, anecdotes, jokes, comics, parables, sayings, crosswords, quotes Add-ons abstracts articles tricks for the curious cribs textbooks basic and additional dictionary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in a textbook, elements of innovation in the lesson, replacing outdated knowledge with new ones Only for teachers perfect lessons calendar plan for the year; methodological recommendations; discussion programs Integrated LessonsThe word aldehyde was coined as an abbreviation of the Latin alcohol dehydrogenatus - dehydrogenated alcohol, the most popular aldehyde is formaldehyde, it is used to make resins, synthesize medicines and as a preservative. The formula of an aldehyde is R-CHO, a compound in which a carbonyl group is combined with hydrogen and a radical.
The word ketone comes from the word acetone, a junior compound in the ketone family. Ketones are used as solvents, drugs, and in the synthesis of polymers. The formula of a ketone is R-C(O)-R, a compound in which a carbonyl group is connected to two radicals.
Structure and properties of the carbonyl group
The carbonyl group is based on the connection of a carbon atom and an oxygen atom through α- and π-bonds. The resonant structure of the group determines the high polarity of the compound and the electron cloud is shifted towards oxygen: C δ+ =O δ- . The introduction of electronegative elements into the bond decreases the polarity, increasing the positive charge of the molecule. Nucleophilic substituents increase the negative charge of oxygen.
The carbon atom in the carbonyl group is a strong electrophile (donates electrons), so most reactions of aldehydes and ketones are carried out by nucleophilic reagents (Lewis bases). Logically, the oxygen atom is a strong nucleophile, and reactions with the oxygen atom are possible using electrophiles (Lewis acids).
Reaction of a carbonyl group with a Lewis base
(R)(R)C δ+ =O δ- + B: → (R)(R)C(B)-O
Reaction of a carbonyl group with a Lewis acid
(R)(R)C δ+ =O δ- + Y: → (R)(R)C-O-Y
In addition, oxygen's unshared electrons give it weak base properties, so those aldehydes and cetones that are insoluble in water dissolve in concentrated sulfuric acid.
Physical properties of the carbonyl group
The high polarity of the C=O bond produces a high dipole moment, causing carboxyl group carriers to have a higher boiling point than hydrocarbons.
The unshared electrons in the oxygen atom form a hydrogen bond with water molecules, therefore, starting with five carbon atoms in the radicals, aldehydes and ketones are poorly soluble in water or not at all.
Aldehydes and ketones with up to 12 carbon atoms are liquids. Aliphatic compounds with a carbonyl group have a density of approximately 0.8, so they float on the surface of water, cyclohexanone has a density of about unity, aromatic aldehydes and ketones have a density slightly greater than the density of water.
Reactions of aldehydes and ketones
Water connection
During the reaction of water with aldehydes and ketones, diols (glycols, dihydric alcohols) are formed. The reaction occurs using a catalyst - an acid or a base and is two-way:
RR-CO + H-OH ↔ R R\ C /OH -OH
Addition of nucleophilic carbons
Important nucleophilic compounds that react with aldehydes and ketones are organometallic compounds (organic compounds in whose molecules there is a bond between a metal atom and a carbon atom/atoms). Some of the representatives of organometallic compounds are Grignard reagents (general formula - R-Mg-X), in reactions with aldehydes and ketones they form alcohols:
RH-C=O + R-C - H 2 -Mg + -Cl - → RH-C-(O-MgCl)(CH 2 -R)
RH-C-(O-MgCl)(CH 2 -R) + H-OH → RH-C-CH 2 R + OH-Mg-Cl
Oxidation of aldehydes and ketones
During oxidation, aldehydes are at an intermediate stage between alcohols and carboxylic acids:
In the presence of hydrogen and oxygen:
R-CH 2 -OH ↔ R-C(=O)-H ↔ R-COOH
Aldehydes are easily oxidized, which allows the use of milder oxidizing agents than simple oxygen. Aromatic aldehydes undergo oxidation more easily than aliphatic ones. The problem with aldehyde oxidation is the formation of by-products.
Ketones are difficult to oxidize; oxidation of ketones requires the use of strong oxidizing agents and a large amount of heat. As a result of oxidation, the C-C bond is broken and an acid is formed (there is an exception):
In the presence of KMnO 4, H and a lot of heat :
CH 3 -C(=O)-CH 2 CH 3 → CH 3 -C(=O)-OH + CH 3 CH 2 -C(=O)-OH
An exception is the oxidation with selenium dioxide, SeO 2, the methyl group following the carbonyl group is oxidized, transforming into another carbonyl group. For example, methyl ethyl ketone is oxidized to diacetyl:
Oxidation of methyl ethyl ketone to diacetyl:
CH 3 CH 2 -C(=O)-CH 3 + SeO 2 → CH 3 -C(=O)-C(=O)-CH 3 + H 2 O + Se
The ease with which aldehydes are oxidized makes it easy to distinguish them from ketones; for this purpose, mild oxidizing agents are used, such as: Tollens' reagent (diammine silver hydroxide, Ag(NH 3) 2 OH), Fehling's reagent (alkaline solution of copper ions Cu in Rochelle salt KNaC 4 H 6 O 6 · 4H 2 O) and Benedict's solution (copper ions with citrate and sodium carbonate). Aromatic aldehydes react with Tollens' reagent, but do not react with Benedict's and Fehling's reagents, which is used to determine the amount of aliphatic and aromatic aldehydes.
Polymerization of aldehydes
Paraldehyde
Acetaldehyde has a boiling point of 20°C, which makes its storage and use difficult. When acetaldehyde is treated with acid at a low temperature, acetaldehyde combines into a cyclic ternary molecule - paraldehyde, with a boiling point of 120°C. Paraldehyde depolymerizes when heated slightly, releasing three molecules of acetaldehyde.
Formaldehyde
For ease of transportation and storage, formaldehyde is sold not in the form of gas, but in the form of formalin - an aqueous solution containing 37-40% paraformaldehyde, OH(CH 2 O) n H, with an average value of n = 30. Paraformaldehyde is a white, amorphous, solid substance obtained by slowly evaporating formaldehyde at low pressure. Polymerization occurs due to the addition of formaldehyde molecules to each other:
CH 2 =O + H 2 O ↔
+ n→ HO-(CH 2 O) n+1 -H
Derlin polymer (polyoxymethylene) is a good linear plastic with a high molecular weight, Derlin has excellent strength and elasticity characteristics.